EQMS Software: The Key to Ensuring Quality and Compliance
In the world of quality and compliance, having an effective system in place for product development is crucial for success.
That’s where eQMS software comes in.
But what exactly is an eQMS?
How does it work?
And why do companies use it?
This blog will dive deep into eQMS software and explore its benefits for ensuring quality and compliance.
We will cover everything from understanding the difference between electronic QMS and enterprise QMS to discovering what makes good eQMS software.
Plus, we will highlight how PERFEQTA can provide a complete 360 view of your quality system.
Get ready to unlock the key to ensuring quality and compliance with eQMS software.
What Is an eQMS?
An eQMS, short for Electronic Quality Management System, is a qms software solution that assists organizations in maintaining and managing quality standards and compliance.
It simplifies operations, automates workflows, centralizes documentation, offers real-time visibility into quality metrics, facilitates efficient tracking of corrective actions, and supports the management of medical device quality.
| Feature | Description |
|---|---|
| Document Control | Manage and control documents throughout their lifecycle, including versioning and approvals. |
| Change Management | Track and manage changes to processes, products, or documents, ensuring compliance. |
| Non-Conformance Management | Capture, investigate, and resolve non-conformances, ensuring quality standards are met. |
| Corrective and Preventive Actions (CAPA) | Identify and address root causes of issues to prevent recurrence and improve processes. |
| Audit Management | Plan, conduct, and track audits to ensure adherence to quality standards and regulations. |
| Risk Management | Identify and assess risks associated with processes and products, implementing mitigation plans. |
| Training Management | Monitor and track employee training to ensure compliance and competency. |
| Supplier Quality Management | Evaluate and manage supplier performance to maintain high-quality materials and services. |
| Complaint Management | Record and manage customer complaints, initiating investigations and resolution processes. |
| Calibration Management | Schedule and track equipment calibration to ensure accuracy and reliability. |
| Analytics and Reporting | Generate insightful reports and analytics on quality performance and trends. |
| Integration | Ability to integrate with other systems like ERP, CRM, or MES for seamless data flow. |
| Mobile Accessibility | Access the EQMS software from mobile devices for on-the-go tasks and approvals. |
| User Permissions | Assign different access levels to users to ensure data security and confidentiality. |
| Regulatory Compliance | Support compliance with relevant industry standards and regulations. |
| Cloud or On-Premises | Choose between a cloud-based or on-premise deployment, depending on your needs. |
What Is the Difference Between Electronic QMS and Enterprise QMS?
Electronic QMS (eQMS) is a digital system for managing quality processes, while Enterprise QMS (EQMS) is a broader term that includes electronic and non-electronic components.
EQMS takes a holistic approach to quality management, encompassing procedures, training programs, and quality control measures beyond digitization.
Why Do Companies Use an eQMS System?
Companies utilize an eQMS system to simplify and optimize their quality management procedures.
This system aids in meeting industry regulations and standards, offering centralized document control for efficient tracking of changes.
Additionally, it provides real-time insights into quality metrics and performance.
How Does an eQMS Work?
An eQMS software solution helps organizations simplify their quality and compliance procedures.
It includes modules for document control, change management, training management, audits, and corrective actions.
Users can create, review, approve, and update documents electronically, ensuring compliance with industry standards and regulations on a centralized platform.
What Makes a Good eQMS Software?
A good eQMS software should be easy to use with a user-friendly interface.
It should have robust document control features to manage quality-related documents effectively.
Automated workflows and notifications should be available to streamline processes. Integration with other systems can improve data accuracy and overall pricing efficiency.
QMS Process Support
Comprehensive support for quality management system (QMS) processes is vital when considering eQMS software.
The ideal software should encompass critical functionalities such as document control, CAPA, non-conformance management, risk management, and audit management.
It should empower users to effortlessly create, review, approve, and distribute documents across the organization while enabling efficient resolution of non-conformances through streamlined CAPA workflows.
Additionally, the software should facilitate risk assessment and management for processes and products and seamlessly offer tools for planning, scheduling, conducting, and documenting audits.
With these capabilities, organizations can ensure the efficacy of their quality management processes and compliance with regulatory standards.
Compliance With FDA 21 CFR Part 11
Compliance with FDA 21 CFR Part 11 is essential in the pharmaceutical industry, ensuring electronic records and signatures’ integrity, authenticity, and confidentiality.
A robust eQMS software, such as PLM, should adhere to this regulation, providing features like user authentication, access controls, audit trails, and electronic signature capabilities.
Companies can streamline their quality management processes by utilizing a compliant eQMS software solution while meeting regulatory requirements and organizing quality events.
Selecting an eQMS software provider offering ongoing support and updates is crucial to ensure sustained compliance with FDA 21 CFR Part 11 regulations.
Software Validation
Validation is an essential part of any reliable eQMS software business process.
It guarantees that the software operates as intended and complies with regulatory standards.
A good eQMS solution should have in-built validation tools and documentation to support the validation process.
It should also offer audit trails and version control, ensuring the integrity of data and adherence to compliance requirements.
Keeping the software validated and updated is crucial for sustained regulatory compliance.
Regulatory Compliance
Regulatory compliance is a critical aspect for organizations looking to implement eQMS software.
A high-quality eQMS system should have built-in features and functionalities that enable organizations to ensure compliance with regulatory standards and requirements.
It should offer tools and processes to manage and track compliance, customizable workflows and templates to streamline compliance processes, and the ability to generate reports and documentation for audits and inspections.
Real-time visibility into compliance status allows organizations to identify and address any non-compliance issues quickly.
Organizations can maintain regulatory compliance while streamlining their quality management processes by choosing a compliance-focused eQMS software provider that offers ongoing support and updates.
Deployment Model
Deployment models for teams’ software offer flexibility and customization options that cater to specific organizational needs.
On-premise team software is installed locally on servers, allowing companies to have control over maintenance and updates.
Cloud-based teams software, hosted by third-party providers, offers scalability, accessibility, and reduced IT burden.
Combining on-premise and cloud-based systems, hybrid solutions can tailor the deployment to fit unique requirements.
These deployment models factor in critical considerations such as data security, financial constraints, and resource availability, ensuring a streamlined quality management process.
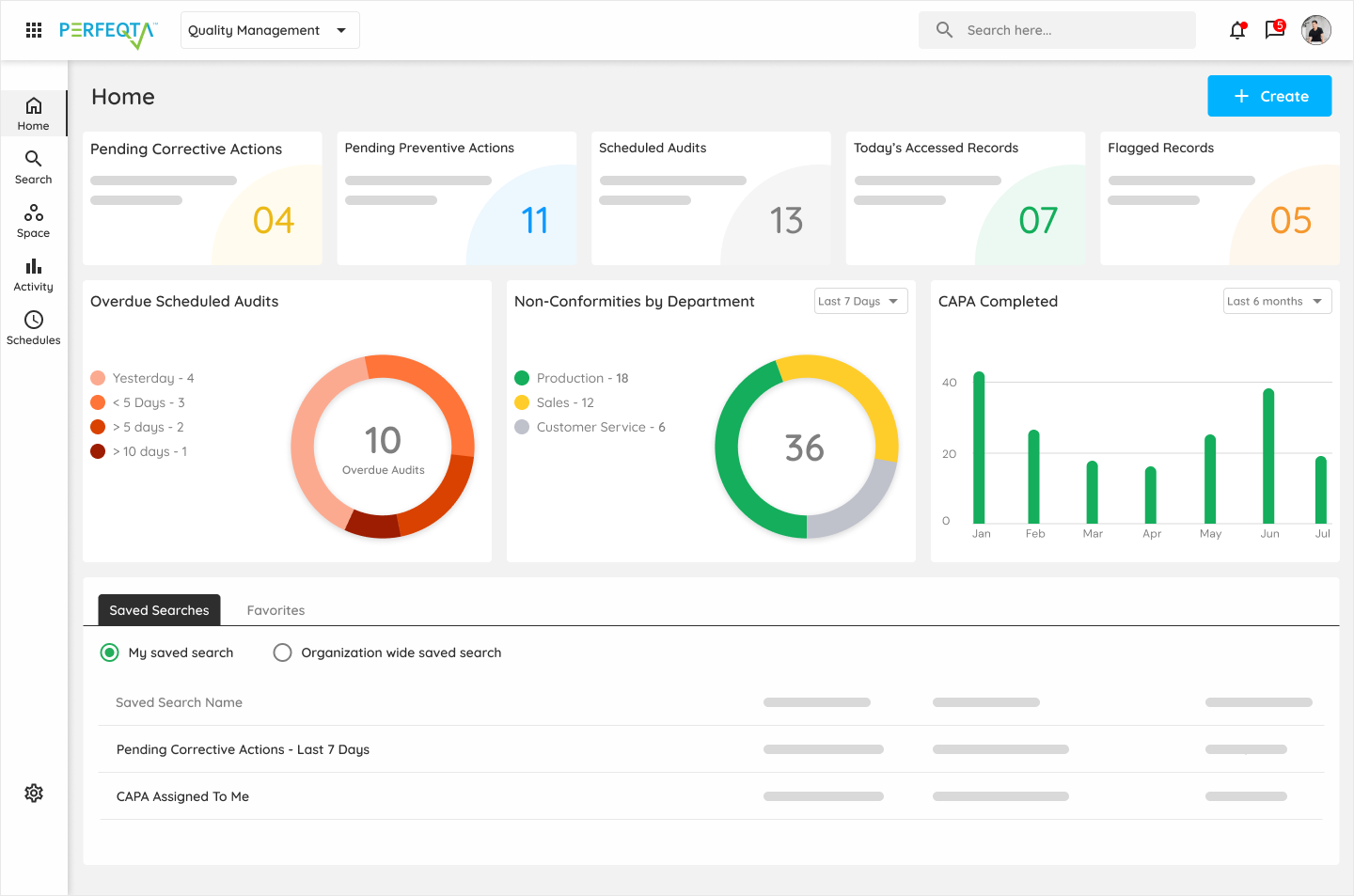
PERFEQTA provides a complete 360 view of your quality system
PERFEQTA is the World’s most flexible QMS & Quality Control Software for life sciences.
It transforms your paper-based system into a paperless system.
PERFEQTA provides a complete 360 view of your quality system, enabling you to streamline and automate your quality management processes.
This EQMS software solution offers a centralized platform for efficiently managing quality documentation, audits, and corrective actions.
With PERFEQTA’s cloud-based approach, you can ensure compliance with regulatory standards and quickly adapt to changing requirements.
The real-time analytics provided by PERFEQTA improve the efficiency of your quality processes and allow for better visibility into quality metrics.
Experience the benefits of PERFEQTA and empower your organization with robust enterprise quality management software.
Product Overview

An overview of quality management software (QMS) and its functionalities is crucial for streamlining and automating quality management processes within an organization.
EQMS software offers a comprehensive solution, providing a centralized platform for managing audits, corrective actions, and more.
Real-time analytics and visibility into quality metrics enable organizations to identify areas for improvement and ensure compliance with regulatory requirements.
Implementing a cloud-based EQMS software enhances efficiency and fosters continuous improvement.
By incorporating features such as change management and audit management, EQMS software allows organizations to streamline their quality processes and achieve the highest levels of product quality.
Document Management
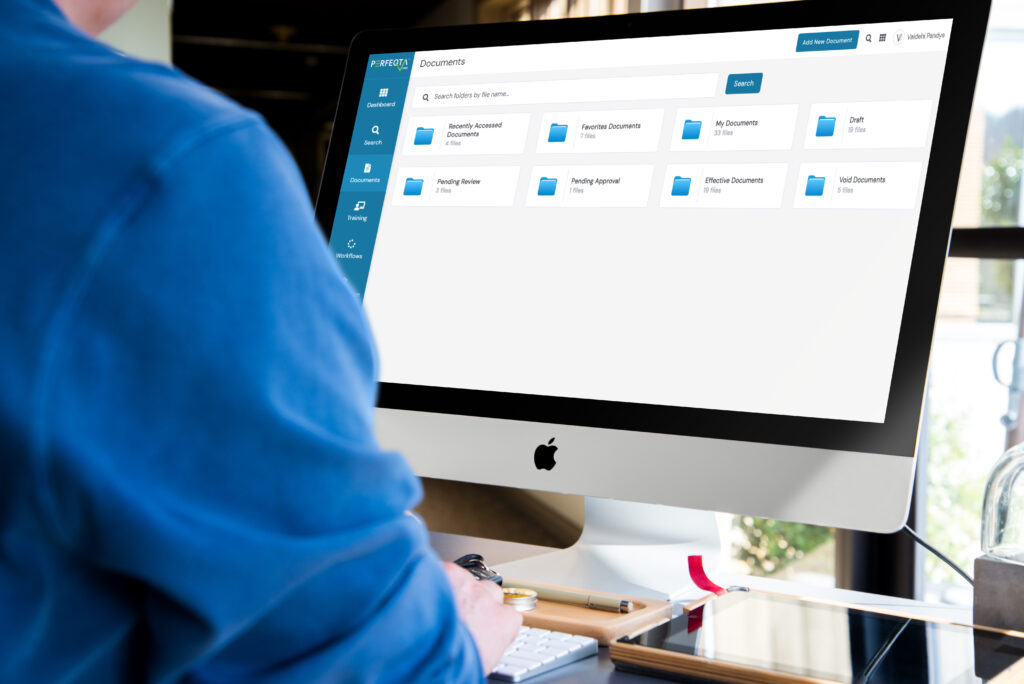
Effective document management is a critical component of quality management for organizations.
EQMS software enables businesses to streamline their document management processes, eliminating manual paperwork and reducing errors.
With EQMS software, organizations can easily track, retrieve, and control important documents, ensuring compliance with industry regulations.
The software facilitates collaboration and document sharing across departments, improving efficiency.
Furthermore, automated alerts and notifications prevent the oversight of crucial document-related deadlines.
Implementing the right EQMS software allows businesses to improve quality control, compliance, and efficiency, enhancing overall performance.
CAPA Management
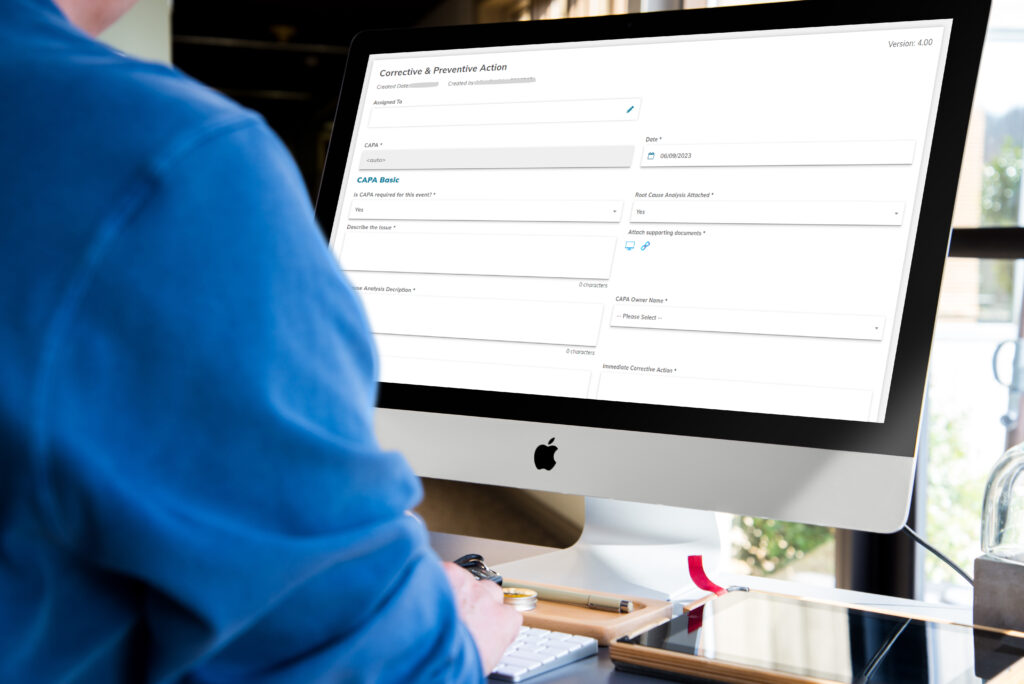
Effective CAPA management is crucial in maintaining quality processes and ensuring compliance with regulatory requirements.
With EQMS software, organizations can streamline their CAPA processes and achieve better visibility and traceability.
By automating the entire process, from capturing nonconformities to implementing corrective and preventive actions, EQMS software enables organizations to address quality issues promptly and prevent their recurrence.
The real-time analytics offered by EQMS software also provides valuable insights into quality data, helping organizations make data-driven decisions for continuous improvement.
Non-conformance Management
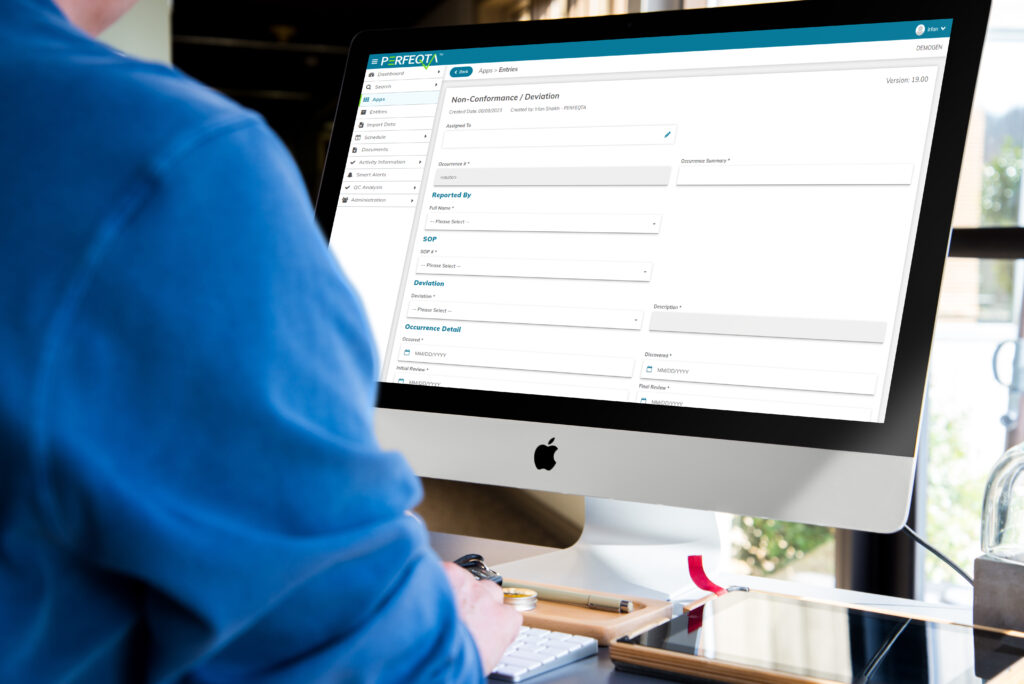
Nonconformance management plays a crucial role in maintaining quality and compliance across industries.
Businesses can use EQMS software to streamline this process, enhancing efficiency and effectiveness.
EQMS software enables easy tracking, investigation, and resolution of nonconformances.
Automated workflows with notifications ensure prompt action.
Additionally, EQMS software provides real-time visibility into non-conformance trends, facilitating proactive measures for prevention.
By enabling regulatory compliance and supporting continuous improvement, EQMS software strengthens quality management processes.
Change Management
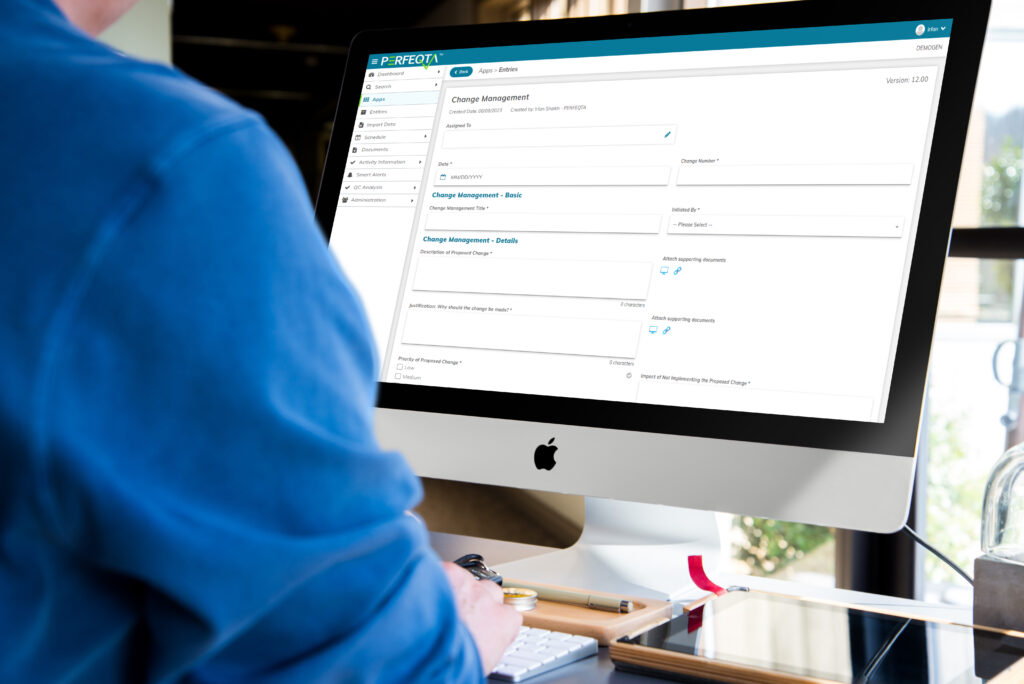
Effective change management is essential for ensuring quality and compliance in organizations.
It involves implementing and managing changes to processes, systems, and procedures in a controlled and efficient manner.
EQMS software streamlines and automates change management processes, minimizing risks and enhancing regulatory compliance.
By leveraging EQMS software, organizations can track and document changes, assign responsibilities, implement approvals, and maintain an audit trail.
This ensures effective implementation of changes and timely identification of potential risks.
With real-time visibility, organizations can monitor progress, address bottlenecks, and make data-driven decisions for continuous improvement.
EQMS software is valuable for optimizing change management processes and driving organizational excellence.
Audit Management
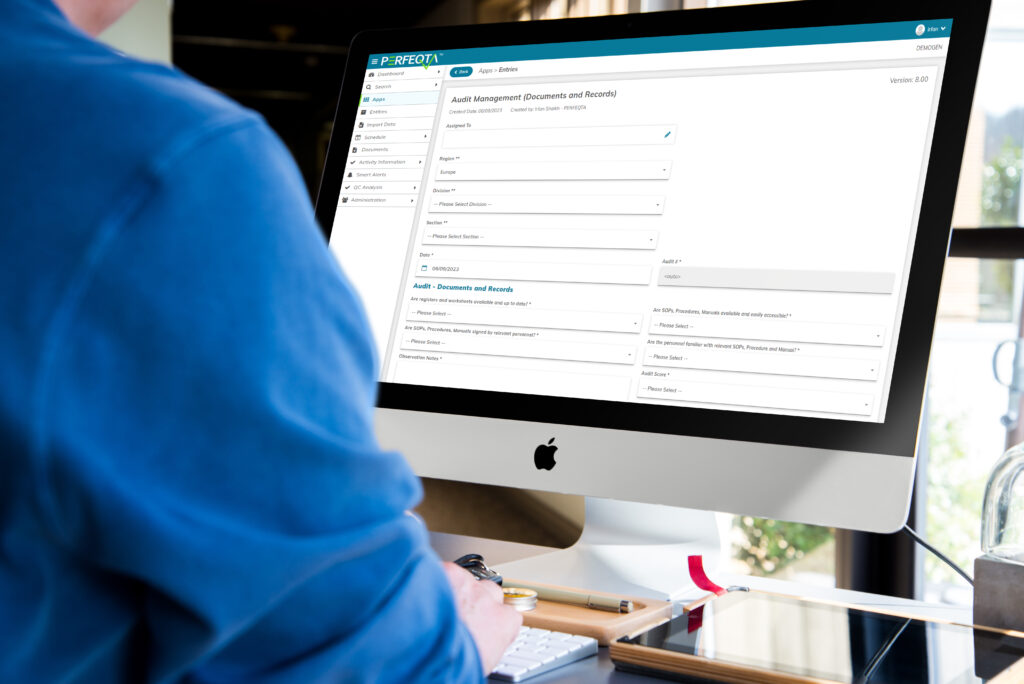
Organizations understand the importance of audit management in ensuring quality and compliance.
It systematically examines and verifies processes to meet regulatory requirements and internal quality standards.
With EQMS software, audit management becomes streamlined and efficient.
EQMS software enables functionalities like document management, audit planning, corrective action management, and real-time visibility into audit processes.
It helps organizations improve their quality management system and proactively enhance compliance.
With features like analytics, document control, and workflows, EQMS software ensures effective audit management.
Supplier Quality Management
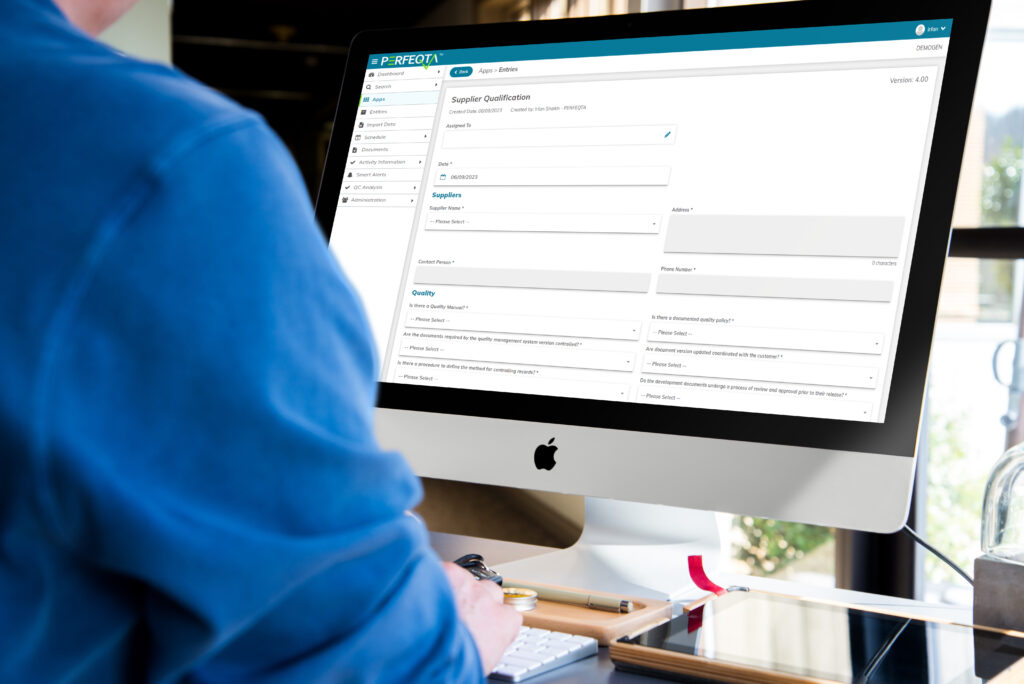
Supplier Quality Management is a critical component of quality and compliance in organizations.
Effectively managing suppliers is essential for tracking and controlling supplier performance, streamlining audits, automating corrective actions, and ensuring transparency in supplier data.
EQMS software plays a vital role in supplier quality management by mitigating the risks associated with non-compliant suppliers and improving communication and collaboration.
It provides real-time visibility into supplier data, enabling better decision-making and strengthening supplier relationships.
With functionalities like supplier audits, performance tracking, and risk mitigation, EQMS software enhances the efficiency and effectiveness of supplier quality management processes.
Training Management
Training management plays a vital role in maintaining quality and compliance in organizations.
It encompasses managing employee training records, certifications, and ongoing training programs.
With the integration of EQMS software, companies can streamline training management processes, ensuring employees receive the necessary training to excel in their roles.
The software tracks training progress, automates notifications, and generates compliance reports. Real-time analytics provide insights into training effectiveness and areas for improvement.
By consolidating training management, organizations achieve regulatory compliance, enhance employee competency, and optimize quality processes.
Complaints Management
Effective management of customer complaints is crucial for ensuring quality and compliance in any organization.
Implementing an EQMS software solution, such as a cloud-based quality management system (QMS) or enterprise quality management software (EQMS), can streamline complaints management and improve overall quality and compliance.
EQMS software allows for the centralized tracking and resolution of customer complaints, ensuring that they are addressed promptly and by regulatory requirements, such as FDA compliance or ISO standards.
It also provides real-time visibility into the complaint data and analytics, enabling organizations to identify and address recurring issues, perform root cause analysis, and implement corrective and preventive actions (CAPA) for continuous improvement.
By leveraging EQMS software for complaints management, organizations can demonstrate their commitment to quality, customer satisfaction, and regulatory compliance.
Maintenance Management
Maintenance Management is vital for the seamless operation of equipment and assets in a business.
It encompasses proactive measures to prevent equipment failures and comply with regulations.
EQMS software facilitates streamlined maintenance processes, task scheduling, and performance metric tracking.
By adopting preventive maintenance strategies, businesses can enhance efficiency, minimize downtime, and mitigate risks.
EQMS software offers real-time visibility, empowering organizations to make informed decisions and prioritize maintenance tasks effectively.
With features like document management, audit management, and change control, EQMS software ensures comprehensive maintenance management without relying on manual and error-prone methods.
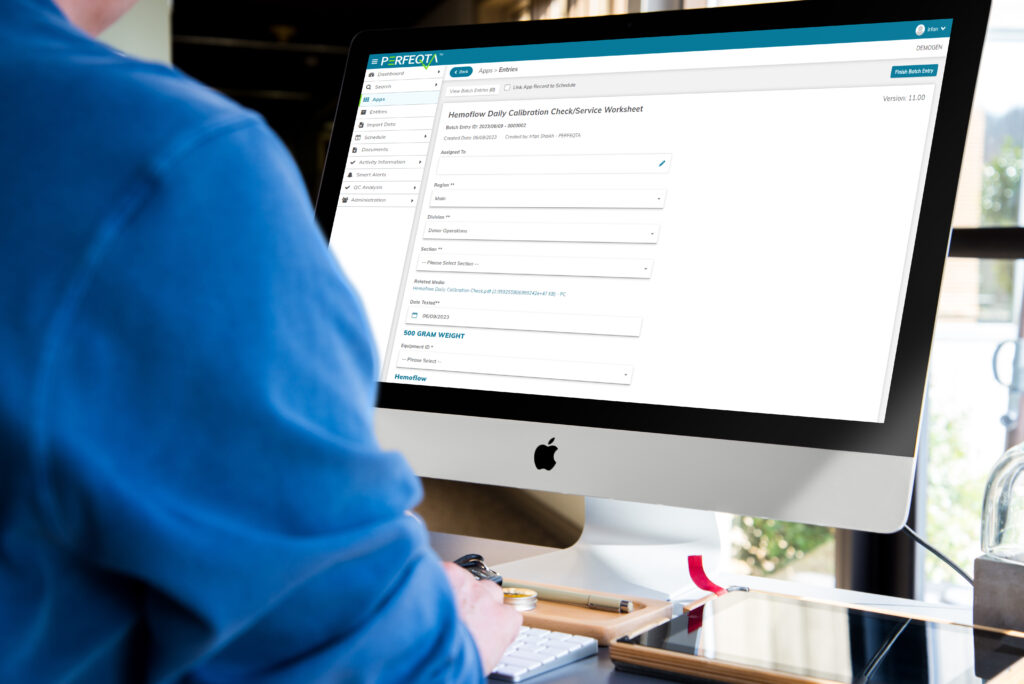
Calibration Management
Calibration Management is essential for maintaining quality and compliance standards in an organization.
By leveraging EQMS software for calibration management, businesses can streamline processes, ensuring adherence to regulations and improving efficiency.
With automated scheduling, tracking, and reporting features, EQMS software simplifies calibration tasks, promoting accuracy and consistency.
Organizations can proactively maintain equipment and assets by utilizing real-time visibility, minimizing non-compliance risk.
EQMS software provides a centralized platform for managing calibration records, generating certificates, and maintaining a comprehensive audit trail to meet regulatory requirements, enhancing traceability and documentation. (Word count: 93)
Inspection Management
Streamlining the inspection process, EQMS software plays a vital role in ensuring quality and compliance.
By automating tasks like scheduling and data collection, it enhances efficiency.
Real-time visibility enables proactive decision-making, while secure storage and centralized documentation ensure compliance with regulations.
Collaboration is improved among stakeholders, fostering communication and accountability. Analytics capabilities allow for data analysis and process improvement.
With its functionalities for inspection management, EQMS software helps organizations maintain quality by regulatory requirements.
Permit Management
Managing permits is essential to meet regulatory standards and comply with regulations.
It encompasses various activities, including construction, environmental impacts, and occupational safety.
EQMS software, a powerful tool for permit management, streamlines processes while reducing manual errors.
It offers automated permit tracking, approval workflows, and document management.
This cloud-based solution enhances visibility, centralizes storage, and improves stakeholder collaboration for proactive decision-making.
By leveraging EQMS software for permit management, organizations can assure compliance, boost productivity, and mitigate risks. Improve your permit management process with an advanced EQMS solution.
Material Compliance Management
Ensuring compliance with material regulations and standards is crucial for industries.
The management of material compliance without EQMS software can be challenging.
However, organizations can streamline and automate material compliance processes by utilizing EQMS software.
This helps in ensuring efficiency and reducing risk.
Key features to look for in EQMS software for effective material compliance management include document management, traceability, and supplier management.
By incorporating EQMS software, organizations can improve compliance with regulatory requirements, enhance their quality management processes, and maintain product quality throughout the lifecycle.
Forms Management
Forms Management is a critical component in maintaining quality and compliance within organizations.
It enables companies to streamline processes, minimize errors, and ensure accurate documentation.
By leveraging EQMS software, organizations can optimize their forms management process.
This software allows for form creation, routing, and approval automation, eliminating the need for manual data entry and minimizing the risk of errors.
EQMS software offers advanced functionalities like version control, electronic signatures, and real-time visibility, ensuring that forms remain updated and compliant with regulatory standards.
Implementing EQMS software for forms management enhances efficiency, traceability, and visibility into quality processes.
Field Safety & Recall Management
Effective Field Safety & Recall Management is essential for ensuring industry regulations and standards compliance.
It is crucial in managing and responding to quality issues, and product recalls.
An advanced EQMS software provides real-time visibility into quality processes, ensuring timely and efficient management.
It streamlines document control, facilitating easy access to updated documentation.
With features such as risk management, product traceability, and audit management, EQMS software enables organizations to identify and resolve potential issues proactively.
Ensure compliance and protect your brand reputation with a robust EQMS solution.
Incident Management
Incident management plays a vital role in maintaining quality and compliance within organizations.
It encompasses the reporting, tracking, and resolution of incidents in an efficient manner.
EQMS software streamlines this process by automating workflows and centralizing data.
Using EQMS software for incident management, organizations can ensure that incidents are appropriately documented and investigated, leading to improved root cause analysis.
This demonstrates a commitment to quality and compliance.
EBR Management
EBR Management is instrumental in regulated industries as it streamlines documentation and record-keeping.
With EBR software, organizations can comply with standards, reducing errors and non-compliance.
This software automates processes, eliminates manual data entry, and reduces paperwork, enhancing efficiency.
Real-time visibility into manufacturing processes improves decision-making and quality control.
Implementing EBR software yields cost savings, improved productivity, and enhanced product quality.
Frequently Asked Questions
How does electronic quality management system (EQMS) software function, and what are its key features?
What benefits can organizations derive from implementing EQMS software besides streamlining quality management and compliance?
How can EQMS software enhance efficiency and productivity, and what factors should be considered when selecting the most suitable solution?
Moreover, what advantages does a cloud-based EQMS offer, and how does including real-time analytics enhance the system’s efficiency?
Conclusion
In conclusion, effective eQMS software is essential for companies to ensure quality and compliance.
It streamlines processes, improves efficiency, and reduces the risk of errors or non-compliance. With features like permit management, material compliance management, forms management, and incident management, PERFEQTA provides a complete 360 view of your quality system.
If you want to experience the benefits of robust eQMS software firsthand, sign up for a free trial/demo/consultation with us today.

