Quality Control
Achieve Quality & Compliance With Ease
With PERFEQTA, you can ensure compliance and quality quickly without compromising your resources.
Trusted by Regulated Organizations
Guarantee success and less stress with everything centralized in one off-the-shelf QMS.
Easy to use
PERFEQTA software is simple to learn and use, so your team can start improving quality control quickly.
Customizable
You can customize PERFEQTA software to fit your unique needs, making it perfect for your business.
Compliance
PERFEQTA software follows industry rules and standards, giving you peace of mind that your quality control is on track.
Real-time insights
PERFEQTA software gives you up-to-date information, so you can catch and fix problems early.
Works with your systems
PERFEQTA software fits smoothly with your other systems, making everything work together.
Helpful support
PERFEQTA team is always ready to help you with any questions or issues you may have.
Saves money
PERFEQTA software helps you save time and resources, which means more money for your business.
Grows with your business
PERFEQTA software can change and grow as your business does, so it always fits your needs.
Benefits of Quality Control Software
Increased productivity of the development team.
Improved Product Quality: Test statistics and defect tracking are more precise and up to date.
Decreased re-work costs as the detection of defects are found earlier in the software project development lifecycle in every stage.
Increased confidence levels in existing product management and future product development.
Increased credibility as the software produced will be highly qualitative.
PERFEQTA Features

What our customers say about us
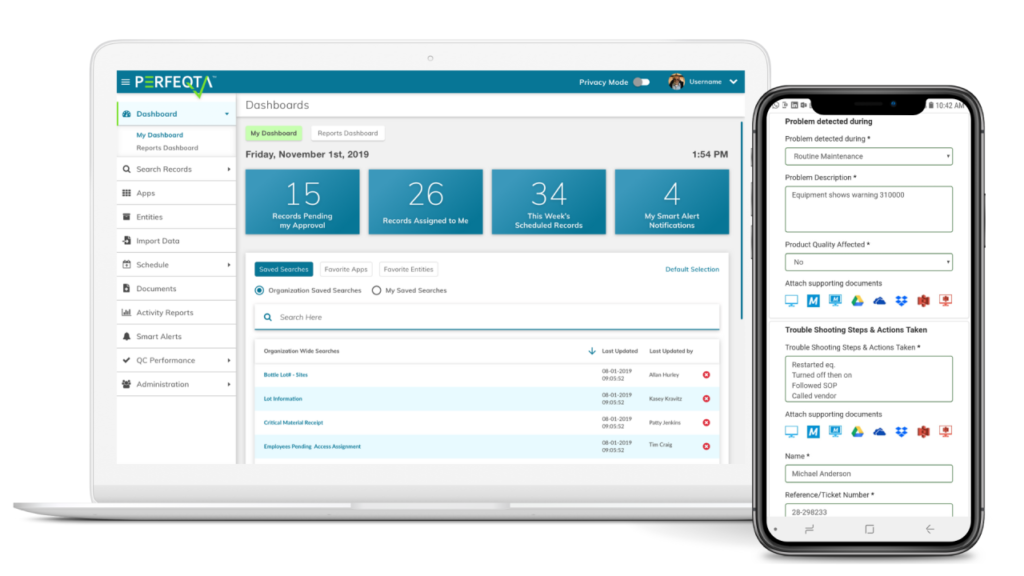
Quality control software customized for your workflow
Track the entire quality control and quality assurance process from start to finish in a single easy-to-use dashboard
Receive access to thousands of quality control, quality management, and business apps including document control, patient care-related apps, safety management, training, equipment management, risk management, risk assessment, CAPA and SCAR processes management, JACHO internal audit applications, and analytical reporting, for regulatory compliance all in one place.
Receive real-time reporting and automatic alerts for quality improvement
Turn findings into actions and actions into positive outcomes.
Be a mobile QC manager with just a few clicks from any device
Nurture a quality-first culture with PERFEQTA
Easily monitor audit management
Ready to simplify compliance and quality while eliminating manual work and paper?











