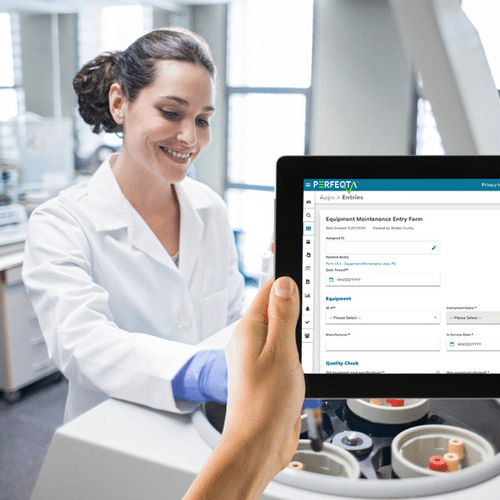
Record, Monitor, and Control Data & Documents Quickly in One Place
We transform your operations for better efficiency, less waste, and easier compliance. QC, QMS, and paperless solutions in minutes!

Smooth Onboarding
We start with a kick-off call, then work closely with you throughout our step-by-step onboarding process.
Easy Migration
Our pre-built templates and streamlined workflows allow you to easily migrate all of your data and workflows.
Training & Support
We’re here to help you throughout the entire process with our personalized support and FREE training.

Do you struggle with:
- Disorganized Papers and forms
- Wasting time with copying and writing the same things
- Headaches from version control
- Keeping up with compliance requirements
- Maintaining Quality
- Faulty equipment
- Auditors finding fault with your procedures
- Managing suppliers
PERFEQTA Helps You Do More
Go 100% paperless and replace your paper forms, excel sheets and rigid software in days. Not weeks or months!
Bring a complete level of ease, efficiency and accuracy from data entry to tracking records to analyzing data and trends in real time.
Easily define access levels across the organization and easily comply with FDA cGMP, CLIA, CMP and ISO regulations and standards with the highest level of traceability, cross-functional visibility, and data security.
Integrated with core quality processes such as document control, nonconformance management, and audit management, it allows you to manage all of your processes in one place.
You can do it all using our drag and drop app builder or use one of our 4000 pre-built templates. No more waiting on technical staff or development teams.
Taking control of your operations and continuous business process improvement should be as easy as using your mouse or finger tips. With PERFEQTA, you can do just that.
Training is minimal and at times, not required. PERFEQTA is intuitive and was built from the ground up to be used without technical background. You will start to see your operations in a different light. Imagine having a wand that will transform the dreading chores of manual data entry, making systems talk to each other and correcting unnecessary mistakes into efficient and automated tasks without constant involvement and never missing a beat.
No extra software or hardware required. You using PERFEQTA can do it in minutes.
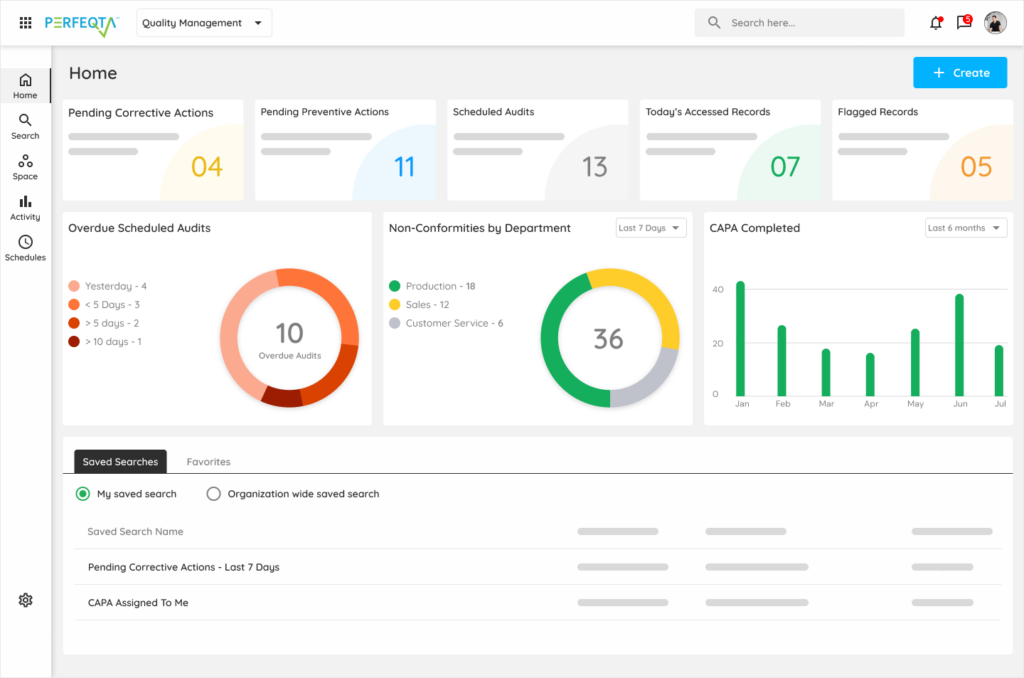
Get the most for your operation from easy to set up and quick to validate and ready to deploy software. Record, track and monitor everything with documentation and traceability. Ensure compliance with GxP, ISO 13485:2016, 21 CFR Part 820, and more.
PERFEQTA goes beyond typical eQMS software in a whole new way. Modules PERFEQTA eQMS covers:
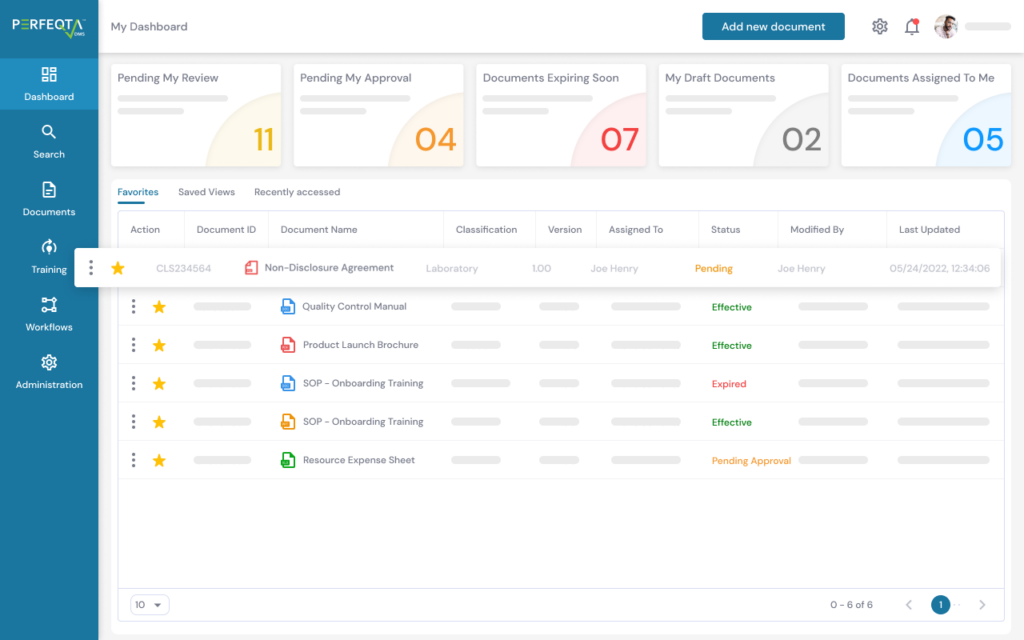
PERFEQTA provides a closed-loop CAPA process to streamline the corrective and preventive action process and ensure compliance with regulations such as GxP, 21 CFR Part 210/211/820, ISO 13485:2016, ISO 9001:2015, ICH Q10, and more.
It also integrates other core QMS modules, such as audit management, non-conformance management, risk management, and more.
The software helps identify trends and track areas of concern through built-in dashboards and reports, making compliance easier.
The system is secure and compliant with FDA 21 CFR Part 11 and EU Annex 11. It provides electronic signatures, time-stamped audit trails, and reporting capabilities.
Users can initiate CAPA procedures from non-conformances/deviations, audit findings, and complaints and monitor quality-related KPIs.
The software also allows users to connect information and relate documents to facilitate the retrieval of needed documentation.
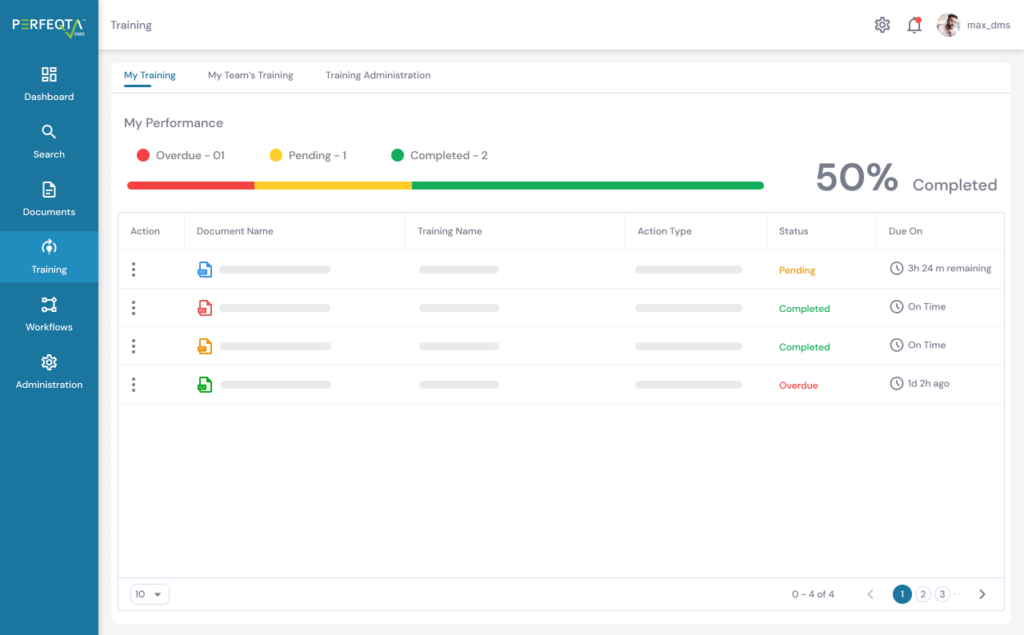
Our Employee Training Management software streamlines your training activities, automating tasks and reminders, and ensuring compliance with training requirements.
Create targeted training groups, implement customized learning rules, and assess training effectiveness with ease.
With our software, employees can easily import classroom or on-the-job training information, assign version-controlled training materials, and track progress through customizable dashboards.
We prioritize on-time training completion, data privacy, and compliance with training management regulations, providing you with a clear overview of your team’s training progress.
Integrate our software with ease, using our API and plug-in solutions to optimize your training management system.
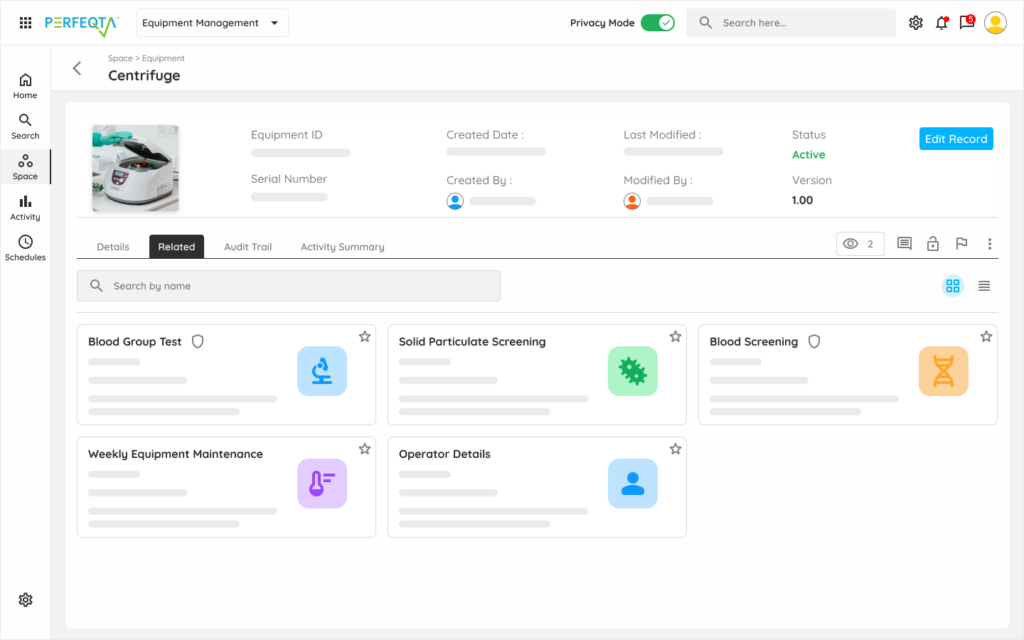
Reduce errors, extend lifetime, reduce costs, and ensure compliance effortlessly with PERFEQTA’s equipment management software.
PERFEQTA is compliant with FDA, GMP/ GAMP, ISO 9001, and 21 CFR Part 11.
Replace your stand-alone equipment calibration and maintenance program for asset tracking, equipment maintenance, inventory management, asset management, with flexible, compliant, and connected equipment management systems for mobile device apps that span the entire life cycle of your equipment.
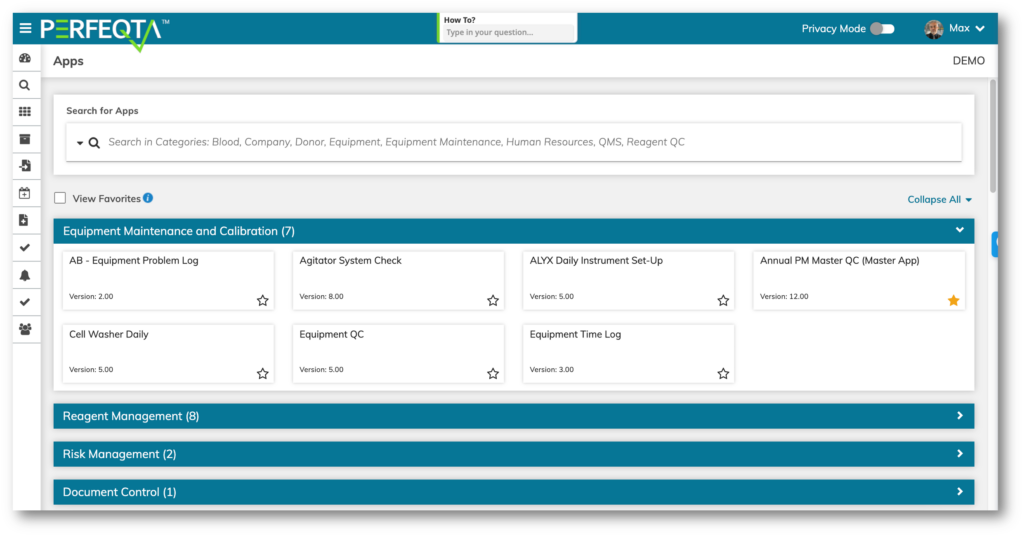
Choose one of our pre-built 4000 templates to automate almost any process in your business beyond QMS and QC.
- Contract Management
- Audit Management
- Competency Assessment
- Quality Event Reporting and Follow-Up
- CAPA
- Non-Conformance and Deviation
- Risk Management
- Assest Management
- Client Relationship Management
- Medical Device Records
- Fleet Management
- Inspections
- Complaints
- Key Performance Indicators (KPI)
- Covid Case and Contract Tracing
- Validation Management
- Recalls Management
- Reviews and Approvals.
PERFEQTA is the only platform that is built around the needs of highly regulated businesses; healthcare, life science, pharmaceutical, biotechnology, blood centers, cell therapy, organ and tissue transplant and procurement, and others.
Use PERFEQTA to rid your organization for dependency on legacy software, worksheets, paper and disconnected databases. Create your own center of truth where all your business processes resides in one software and connects to all data sources; ERP, EMR, BECS, LIS, CRM, HRMS and other enterprise software.
PERFEQTA is more than QMS. It is a platform to transform your business and take advantage of every improvement opportunity without the need for extensive software development, costly upgrades and length validation cycles.
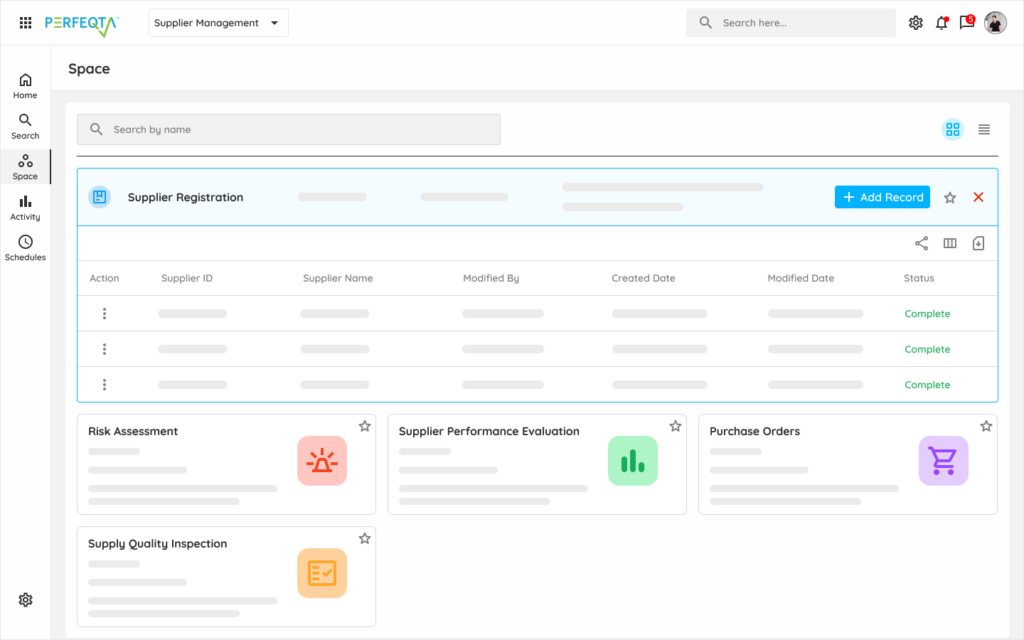
Our supplier management software simplifies supplier qualification, performance monitoring, related incidents, and audit processes.
Integrated with core quality processes such as document control, nonconformance management, and audit management, it allows you to manage all supplier records in one place.
Flexible and easy-to-use, our supplier management module enables you to streamline your supplier management processes, ensuring compliance and optimizing supplier performance.
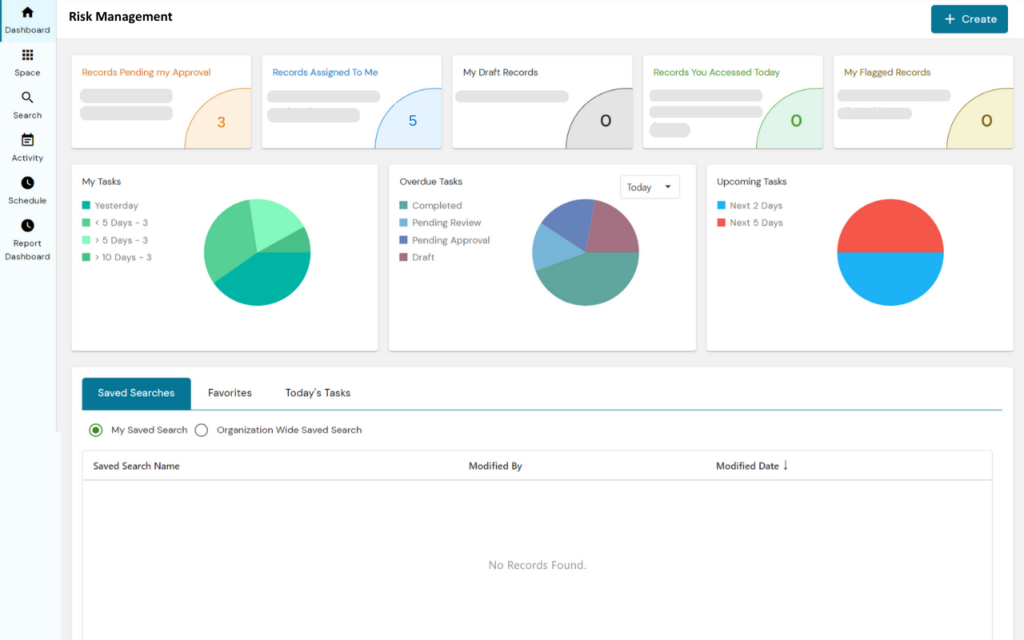
PERFEQTA, is a risk management software that allows companies in the life science industry to consolidate their risk management files into one traceable system.
The software can link risk management documentation with products, suppliers, customers, and equipment, and provides dashboards for an accurate representation of risk across products and processes.
PERFEQTA streamlines the risk control process by creating a risk management plan and hazard analysis as part of the design control process, and enables users to stay in compliance with ISO 14971:2019 risk management procedures and forms.
The software helps develop risk management habits across the organization by creating and maintaining Risk Management SOPs that capture training records for employees.
PERFEQTA also offers additional modules for training management, CAPA management, complaint management, change management, design control, document control, equipment calibration, electronic signatures, product lifecycle management, audit management, deviation management, supplier management, form management, electronic batch records, and nonconformance management.
What our customers say about us
Trusted by Regulated Organizations
Package & Pricing
Launch
Starts at $800 per month
Streamline your paperless journey and replace clunky software with essential features and mobile access.
Growth
Starts at $1500 per month
Expand with advanced workflows, analytics, integrations, and offline access.
Enterprise
Custom Pricing
Comprehensive, AI-powered with full mobile access and enterprise-level security and customization.
How it Works

1. Get Ready To Pass Audits, Stress-Free
2. Respond to any change – quickly


3. Never Miss An Event Or Action Ever
4. Fully Integrated & Cost-Effective


5. Mobile Access
Who We Are
We are a team with a common passion: technology is a right of passage for humanity. We love before/after transformations and we want to make technology available to all organizations without the crazy cost, the steep learning curve and the time to implement and deploy. We focus on highly regulated organizations that must comply with FDA, CAP, CLIA, HIPAA, GxP, GAMP 5, and ISO regulations and standards.












