5 Steps To Minimize Non-Conformance And Improve Your Operations

In this article, we will discuss what non-conformance is, the types of non-conformance in different industries, the Impact of non-conformance on business operations and reputation, Identifying the root cause of non-conformance, steps to prevent non-conformance from occurring again, the importance of corrective and preventive actions, documentation and reporting of non-conformance incidents, ways to improve quality control process to prevent non-conformance, benefits of implementing the non-conformance management system, training and education for employees on how to identify and report non-conformance incidents, the importance of digital tools like non-conformance management software
Understanding what non-conformance means
Non-conformance refers to a situation where something does not meet the expected standards, specifications, or requirements.
This can occur in many different contexts, from manufacturing and construction to quality control and safety regulations.
Understanding what non-conformance means is important because it allows us to identify areas where improvements can be made and take action to prevent future occurrences.
By addressing non-conformances as they arise, we can ensure that our products or services are of the highest quality and meet the expectations of our customers.
So if you come across a non-conformance in your work or personal life, don’t panic! Instead, take the time to investigate the issue and determine what steps need to be taken to correct it.
With a little effort and attention to detail, you can turn a non-conformance into an opportunity for growth and improvement.
Types of non-conformance in different industries
To correct an organization’s course, there are two types of non-conformances to consider: minor non-conformances and major non-conformances. The former requires fewer measures to resolve than the latter.
ISO 9001 is an international standard that outlines the requirements for a quality management system. One of the key components of ISO 9001 is the identification and management of non-conformities. Non-conformities can be major or minor, depending on their severity.
A major non-conformity is a serious breach of the ISO 9001 standard and indicates a significant failure in the quality management system. Major non-conformities must be addressed immediately, and corrective action must be taken to prevent recurrence.
Minor non-conformities, on the other hand, are less severe and do not pose a significant risk to the quality management system. They should still be addressed and corrected, but they do not require immediate action.
It is important for organizations to identify and address any non-conformities in their quality management system in order to ensure consistent delivery of high-quality products and services. By effectively managing non-conformities, organizations can improve customer satisfaction, reduce waste, and increase efficiency.
There are various types of non-conformances that can occur in different industries, such as manufacturing, healthcare, and construction.
In manufacturing, non-conformance can occur during the production process when a product does not meet the specified quality standards.
This can include issues with dimensions, material composition, or functionality.
In healthcare, non-conformance can refer to errors in patient care, such as medication errors or misdiagnosis.
It can also include issues related to documentation and record-keeping.
In the construction industry, non-conformance can occur when building materials or methods do not meet the required codes and regulations.
This can result in safety hazards or structural issues.
Regardless of the industry, it is important to identify and address non-conformances promptly to prevent further issues and ensure compliance with established standards.
Nonconformity is the failure to meet one or more of the existing requirements in ISO 9001.The impact of non-conformance on business operations and reputation
Non-conformance can have a significant impact on business operations and reputation.
When a product or service does not meet expected standards, it can result in delays, wasted resources, and lost revenue.
Additionally, non-conformance can damage a company’s reputation, leading to decreased customer trust and loyalty.
To mitigate the impact of non-conformance, companies should prioritize quality control measures, such as regular inspections and testing.
It is also important to have clear communication channels between employees to ensure that issues are identified and addressed promptly.
By taking proactive steps to prevent non-conformance, businesses can protect their operations and reputation while also improving overall efficiency and effectiveness.
Here’s a sample table for recording non conformance:
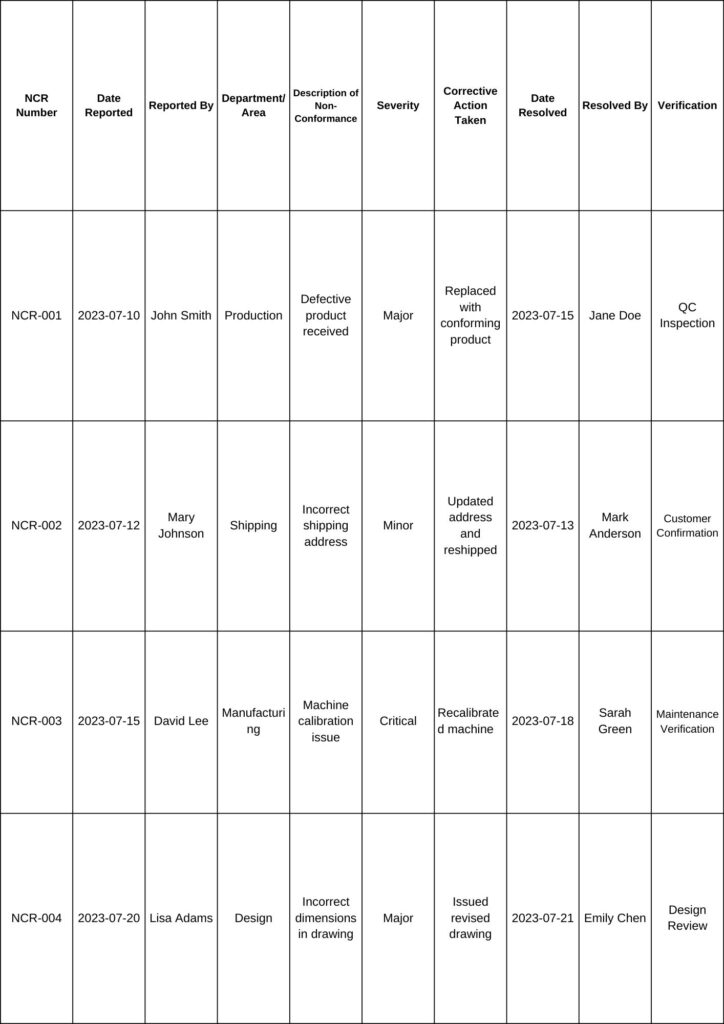
Note:
- NCR Number: A unique identifier for each non conformance report.
- Date Reported: The date when the non-conformance was identified and reported.
- Reported By: Name of the person who reported the non conformance.
- Department/Area: The department or area where the non conformance occurred.
- Description of Non-Conformance: A brief description of the issue or problem identified.
- Severity: The severity level of the non conformance (e.g., Minor, Major, Critical).
- Corrective Action Taken: The action taken to address and rectify the non-conformance.
- Date Resolved: The date when the non conformance was resolved.
- Resolved By: Name of the person or team responsible for resolving the non conformance.
- Verification: The method used to verify that the corrective action was successful (e.g., QC Inspection, Customer Confirmation, Maintenance Verification, Design Review, etc.).
Please note that the actual structure of the table may vary based on the specific requirements and needs of your organization.
You can modify the table to include any additional fields or data that are relevant to your non-conformance reporting process.
Identifying the root cause of non-conformance
Identifying the root cause of non-conformance is an essential step in ensuring quality control and preventing future issues.
When a non-conformance occurs, it’s important to investigate and determine the underlying cause.
This can involve reviewing procedures, equipment, materials, training processes, or any number of factors that may have contributed to the issue.
By identifying the root cause, you can take corrective action to prevent similar problems from occurring in the future.
It’s also important to focus on creating a culture of continuous improvement, where teams are encouraged to identify potential issues and suggest solutions.
By working together and staying vigilant, we can ensure that our products and services meet the highest standards of quality and customer satisfaction.
Steps to prevent non-conformance from occurring again
Preventing non-conformance from occurring again is essential for maintaining the quality of products and services.
Here are some steps that can be taken to prevent non-conformance from happening in the future:
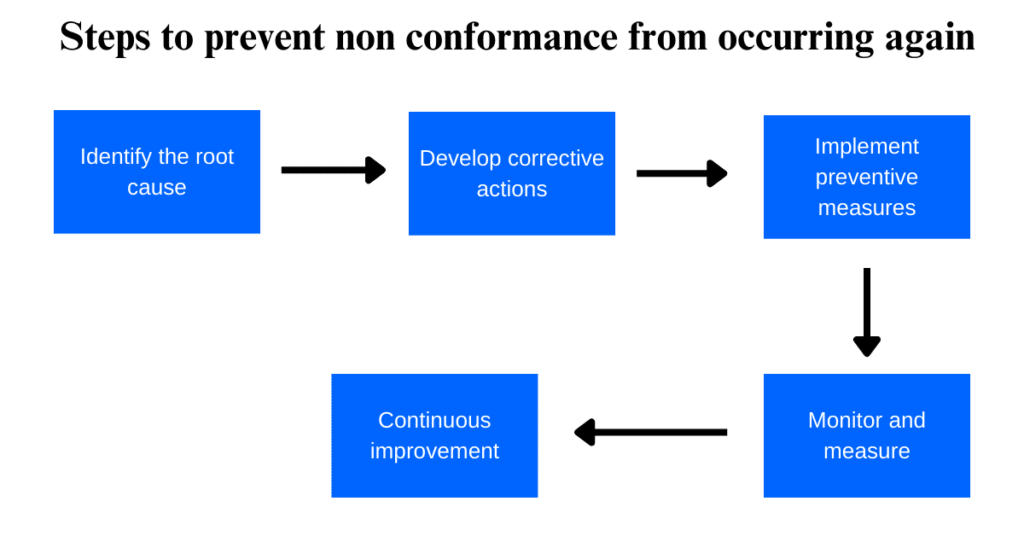
1. Identify the root cause:
It is important to identify the root cause of non-conformance to prevent it from happening again. This can be done by analyzing data and conducting a thorough investigation.
2. Develop corrective actions:
Once the root cause has been identified, it’s time to develop corrective actions that will address the issue and prevent it from happening again.
3. Implement preventive measures:
Take preventive measures to ensure that non-conformance does not occur again. This may include training employees, improving processes, or implementing new quality control procedures.
4. Monitor and measure:
Keep track of progress by monitoring and measuring the effectiveness of corrective and preventive actions taken.
5. Continuous improvement:
Continuous improvement is key to preventing non-conformance in the future. Regularly review processes and procedures to identify areas for improvement and implement changes accordingly.
By following these steps, businesses can effectively prevent non-conformance from occurring again while also improving overall quality control processes.
Importance of corrective and preventive actions
Corrective and preventive actions (CAPA) are essential for ensuring the quality of products and processes in any organization.
Corrective actions address existing problems or issues that have been identified, while preventive actions aim to identify potential issues before they occur.
By implementing CAPA processes, organizations can improve their overall performance by addressing quality issues at the source and preventing them from recurring.
Corrective and preventive actions are important because they help to reduce waste, improve efficiency, and increase customer satisfaction.
When a problem is addressed through corrective action, it helps to identify the root cause of the issue so that it can be prevented from happening again.
This not only improves product quality but also saves time and resources in the long run.
Preventive actions are equally important because they help to identify potential issues before they occur.
By anticipating problems, organizations can take steps to prevent them from happening in the first place.
This can lead to significant improvements in efficiency, cost savings, and customer satisfaction.
Overall, implementing effective corrective and preventive actions is critical for any organization that wants to maintain high levels of quality and ensure customer satisfaction.
By addressing issues proactively and continuously improving processes, organizations can stay ahead of the curve and deliver exceptional products and services to their customers.
‘Non-conformity’ is included in all 3 standards, and all need ‘corrective action’.Documentation and reporting of non-conformance incidents
When it comes to documenting and reporting non-conformance incidents, it’s important to have a clear and organized approach.
Start by creating a template or form for the non-conformance report (NCR) that includes all the relevant information, such as the date, time, location, description of the incident, and any contributing factors.
This will help ensure consistency in reporting and make it easier to identify trends or patterns over time.
It’s also important to encourage open communication among team members so that everyone feels comfortable reporting incidents without fear of retribution.
This can be achieved by emphasizing the importance of learning from mistakes and continuously improving processes.
When documenting incidents, be sure to include any corrective actions taken and follow up on them to ensure they have been effective.
Regularly reviewing incident reports can also help identify areas for improvement and prevent similar incidents from occurring in the future.
Remember, documentation and reporting of non-conformance incidents are essential for maintaining quality standards and ensuring continuous improvement.
So let’s work together to create a culture of transparency and accountability where everyone feels empowered to report incidents and contribute to the success of our organization.
Ways to improve quality control processes to prevent non-conformance
Improving quality control processes is crucial for preventing non-conformance and ensuring that products meet industry standards. Here are a few tips to help improve your quality control processes:
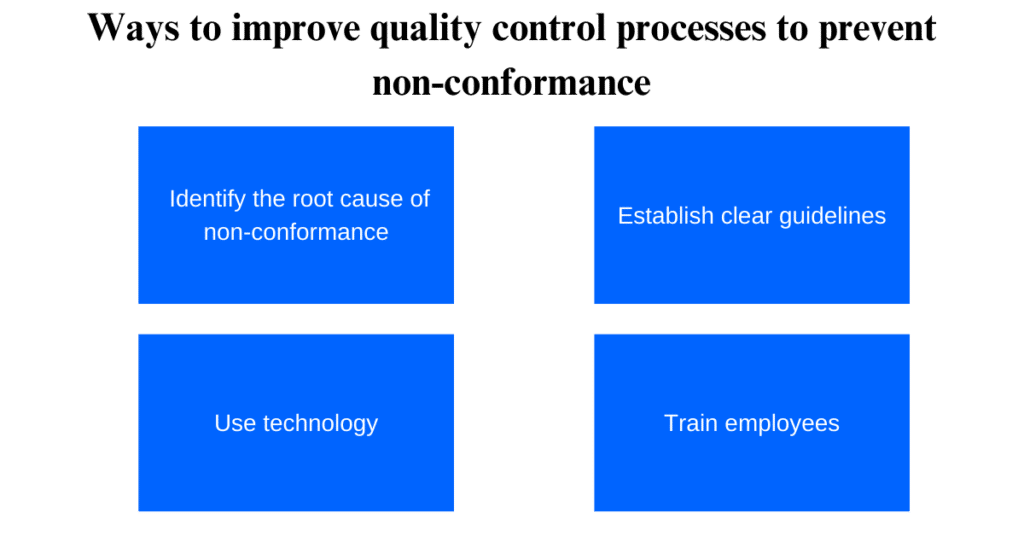
1. Identify the root cause of non-conformance:
This will allow you to identify areas that need improvement and make changes to prevent issues from occurring in the future.
2. Establish clear guidelines:
Clearly defining all quality control procedures can help ensure consistency and accuracy across all aspects of production.
3. Use technology:
Incorporating technology such as automated inspection equipment can help detect defects quickly and accurately, reducing the likelihood of non-conformance. PERFEQTA software helps you to automate all these processes without wasting time, money, and effort.
4. Train employees:
Providing comprehensive training to employees on quality control processes can increase their understanding of the importance of maintaining high standards and reduce the likelihood of errors or oversights.
Remember, improving your quality control processes is an ongoing effort that requires continuous evaluation and adjustments. By implementing these tips, you can create a culture of excellence that prioritizes quality and ensures customer satisfaction.
Benefits of implementing a non-conformance management system
Implementing a non-conformance management system can bring numerous benefits to an organization.
Firstly, it helps in identifying and correcting any issues or problems that may arise during the production process.
This not only increases efficiency but also reduces the risk of defects and errors in the final product.
Secondly, it ensures compliance with industry regulations and standards, which is crucial for maintaining customer confidence and trust.
Thirdly, a nonconformance management system encourages continuous improvement by providing valuable insights into areas that require attention and resources.
It also helps in identifying potential risks and opportunities for improvement.
Finally, it promotes a culture of accountability, where employees are encouraged to take ownership of their work and contribute to the overall success of the organization.
Overall, implementing a nonconformance management system can lead to increased productivity, improved quality, and enhanced customer satisfaction.
Training and education for employees on how to identify and report non-conformance incidents
As a company, it’s important to make sure that all employees are trained and educated on how to identify and report non-conformance incidents.
This not only helps maintain a safe and efficient work environment but also ensures that any potential issues are addressed in a timely manner.
So, what exactly does this training entail?
Well, employees will learn how to recognize non-conformance incidents, such as defects in products or machinery malfunctions.
They will also learn how to properly report these incidents, including who to notify and what information to provide.
By providing this training, we can empower our employees with the knowledge and tools they need to help maintain a high standard of quality for our products and services.
And remember, if you ever have any questions or concerns about identifying or reporting non-conformance incidents, don’t hesitate to reach out to your supervisor or HR representative for assistance.
Importance of Digital Tools Like Non-Conformance Management Software
Digital tools like non-conformance management software have become increasingly important in today’s fast-paced business environment.
These tools provide a streamlined way for organizations to manage and track non-conformances, ensuring that issues are identified and resolved quickly and efficiently.
One of the key advantages of using non-conformance management software is that it provides real-time visibility into the status of non-conformances, allowing organizations to take proactive steps to address any issues before they escalate.
In addition, these tools can help to improve collaboration between teams and departments, as all stakeholders have access to the same information and can work together to resolve any issues.
Overall, digital tools like non-conformance management software are an essential part of any organization’s quality management system in today’s digital age.
The PERFEQTA Non-Conformance Management Software complies with ISO 9001:2015, ISO 13485:2016, and FDA 21 CFR Part 820 QMS standards.
