CAPA & NONCONFORMANCES
Streamline your CAPA with PERFEQTA
Identify, Track, and Resolve Nonconformances with Ease

“It was a no-brainer for us to implement PERFEQTA.”
~ Wendy Trivisonno
President and CEO, National Blood Testing Cooperative
Trusted by Regulated Organizations

Simplify your CAPA management processes
Ensure compliance with industry regulations
Improve overall efficiency and productivity
Achieve a culture of quality and continuous improvement

Go From Uncertain to 100% Confident
Streamline Your CAPA Process in Simple Steps with PERFEQTA
Identify Nonconformances
Quickly identify and track nonconformances, assign tasks to team members, and set priorities.
Investigate and Analyze
Manage investigations and root cause analyses to determine the underlying cause of nonconformances. Involve all team members in the process to ensure that everyone is aligned towards a common goal.
Assign and Monitor Actions
Assign corrective and preventive actions, set due dates, and track progress to ensure that nonconformances are resolved effectively and efficiently.
Track CAPA Effectiveness
Monitor the effectiveness of corrective and preventive actions over time to ensure that nonconformances do not recur.
Manage Documents
Manage all CAPA-related documents, procedures, and tasks in a centralized location. No more searching through multiple folders to find the right document.
Automated Escalation Notifications
Receive automated notifications when nonconformances require immediate attention or are overdue, ensuring timely resolution and reducing the risk of further issues or noncompliance.
Why PERFEQTA
Comprehensive and Integrated Solution
PERFEQTA offers a complete solution for CAPA management, as well as other quality management processes such as audit management, document control, training management, and supplier management.
Easy to Use
Our intuitive interface makes it easy for team members to use and understand.
Cloud-Based
Our software is cloud-based, meaning that you can access it from anywhere and on any device.
Exceptional Customer Support
Our dedicated support team is available to assist you with any questions or issues you may have.
How PERFEQTA Helps You Do More
Go 100% paperless and replace your paper forms, excel sheets and rigid software in days. Not weeks or months!
Bring a complete level of ease, efficiency and accuracy from data entry to tracking records to analyzing data and trends in real time.
Easily define access levels across the organization and easily comply with FDA cGMP, CLIA, CMP and ISO regulations and standards with the highest level of traceability, cross-functional visibility, and data security.
Integrated with core quality processes such as document control, nonconformance management, and audit management, it allows you to manage all of your processes in one place.
You can do it all using our drag and drop app builder or use one of our 4000 pre-built templates. No more waiting on technical staff or development teams.
Taking control of your operations and continuous business process improvement should be as easy as using your mouse or finger tips. With PERFEQTA, you can do just that.
Training is minimal and at times, not required. PERFEQTA is intuitive and was built from the ground up to be used without technical background. You will start to see your operations in a different light. Imagine having a wand that will transform the dreading chores of manual data entry, making systems talk to each other and correcting unnecessary mistakes into efficient and automated tasks without constant involvement and never missing a beat.
No extra software or hardware required. You using PERFEQTA can do it in minutes.
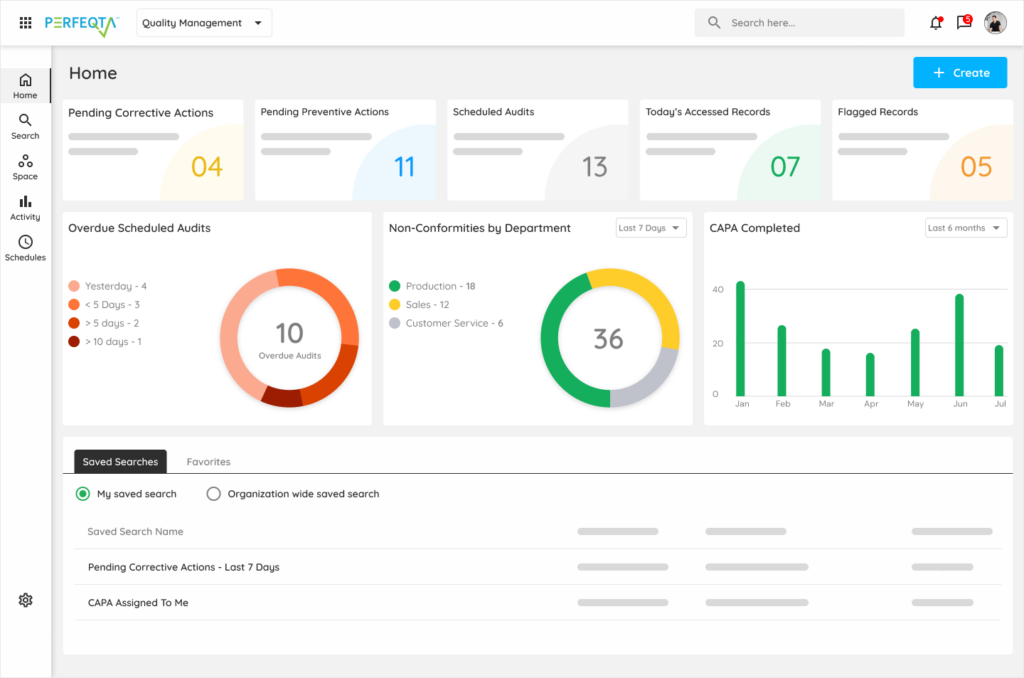
Get the most for your operation from easy to set up and quick to validate and ready to deploy software. Record, track and monitor everything with documentation and traceability. Ensure compliance with GxP, ISO 13485:2016, 21 CFR Part 820, and more.
PERFEQTA goes beyond typical eQMS software in a whole new way. Modules PERFEQTA eQMS covers:
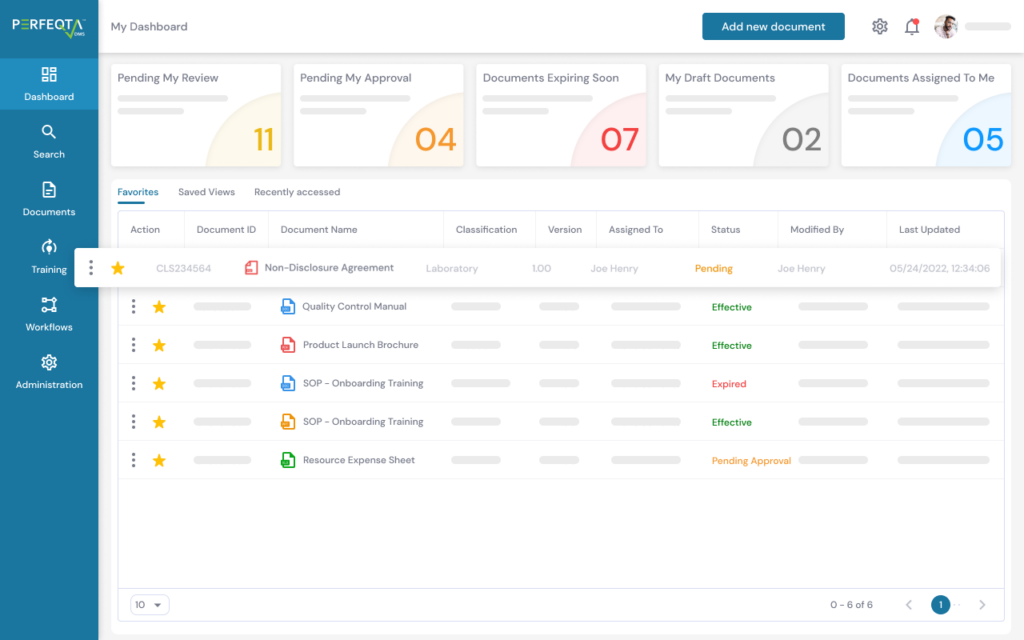
PERFEQTA provides a closed-loop CAPA process to streamline the corrective and preventive action process and ensure compliance with regulations such as GxP, 21 CFR Part 210/211/820, ISO 13485:2016, ISO 9001:2015, ICH Q10, and more.
It also integrates other core QMS modules, such as audit management, non-conformance management, risk management, and more.
The software helps identify trends and track areas of concern through built-in dashboards and reports, making compliance easier.
The system is secure and compliant with FDA 21 CFR Part 11 and EU Annex 11. It provides electronic signatures, time-stamped audit trails, and reporting capabilities.
Users can initiate CAPA procedures from non-conformances/deviations, audit findings, and complaints and monitor quality-related KPIs.
The software also allows users to connect information and relate documents to facilitate the retrieval of needed documentation.
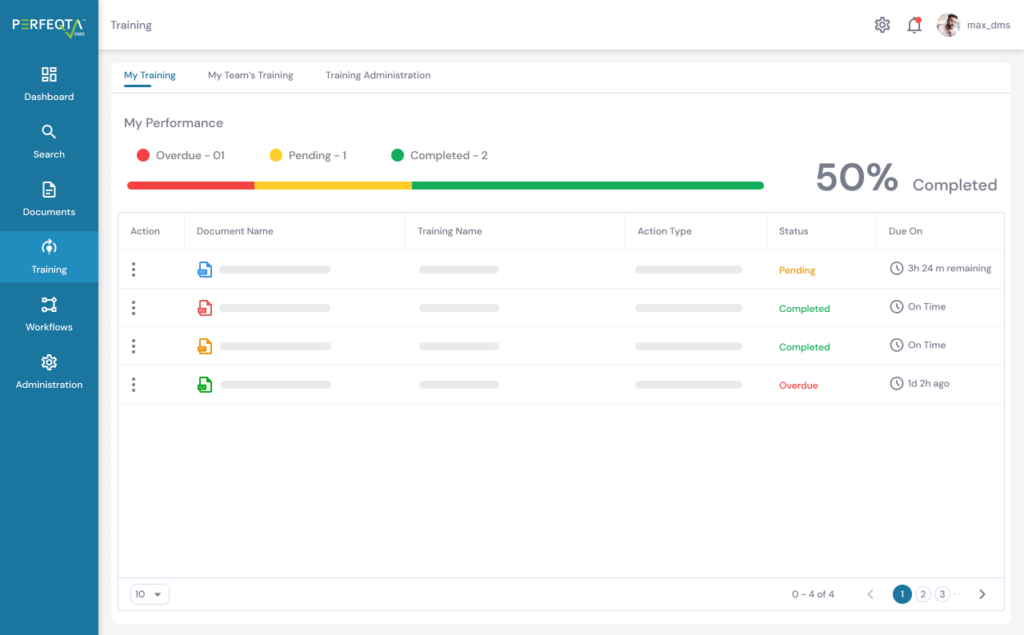
Our Employee Training Management software streamlines your training activities, automating tasks and reminders, and ensuring compliance with training requirements.
Create targeted training groups, implement customized learning rules, and assess training effectiveness with ease.
With our software, employees can easily import classroom or on-the-job training information, assign version-controlled training materials, and track progress through customizable dashboards.
We prioritize on-time training completion, data privacy, and compliance with training management regulations, providing you with a clear overview of your team’s training progress.
Integrate our software with ease, using our API and plug-in solutions to optimize your training management system.
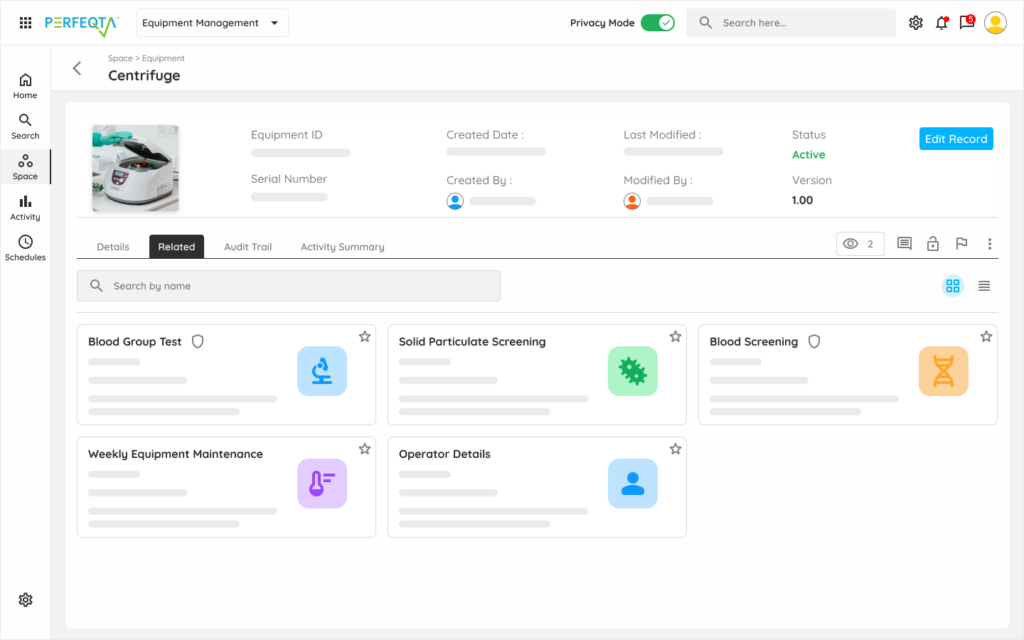
Reduce errors, extend lifetime, reduce costs, and ensure compliance effortlessly with PERFEQTA’s equipment management software.
PERFEQTA is compliant with FDA, GMP/ GAMP, ISO 9001, and 21 CFR Part 11.
Replace your stand-alone equipment calibration and maintenance program for asset tracking, equipment maintenance, inventory management, asset management, with flexible, compliant, and connected equipment management systems for mobile device apps that span the entire life cycle of your equipment.
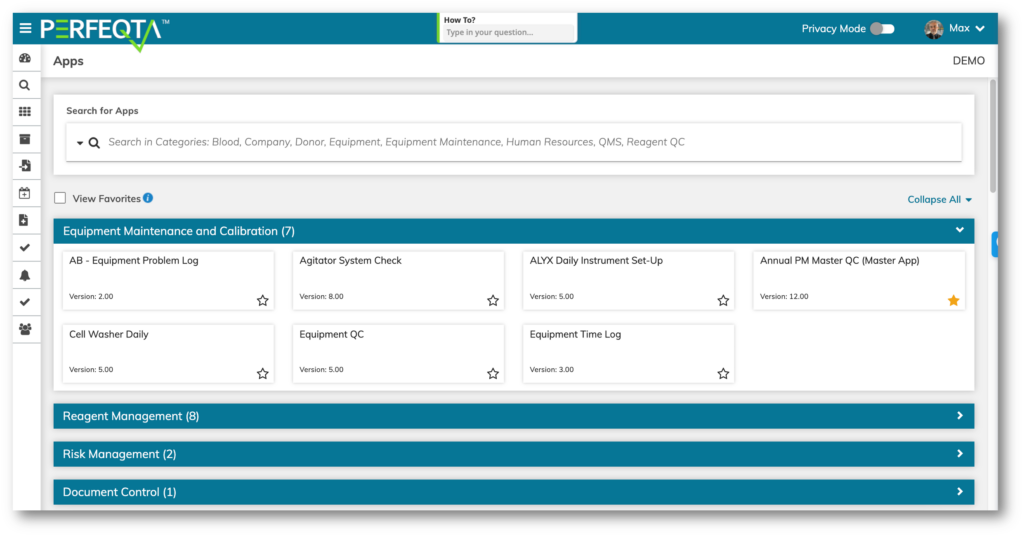
Choose one of our pre-built 4000 templates to automate almost any process in your business beyond QMS and QC.
- Contract Management
- Audit Management
- Competency Assessment
- Quality Event Reporting and Follow-Up
- CAPA
- Non-Conformance and Deviation
- Risk Management
- Assest Management
- Client Relationship Management
- Medical Device Records
- Fleet Management
- Inspections
- Complaints
- Key Performance Indicators (KPI)
- Covid Case and Contract Tracing
- Validation Management
- Recalls Management
- Reviews and Approvals.
PERFEQTA is the only platform that is built around the needs of highly regulated businesses; healthcare, life science, pharmaceutical, biotechnology, blood centers, cell therapy, organ and tissue transplant and procurement, and others.
Use PERFEQTA to rid your organization for dependency on legacy software, worksheets, paper and disconnected databases. Create your own center of truth where all your business processes resides in one software and connects to all data sources; ERP, EMR, BECS, LIS, CRM, HRMS and other enterprise software.
PERFEQTA is more than QMS. It is a platform to transform your business and take advantage of every improvement opportunity without the need for extensive software development, costly upgrades and length validation cycles.
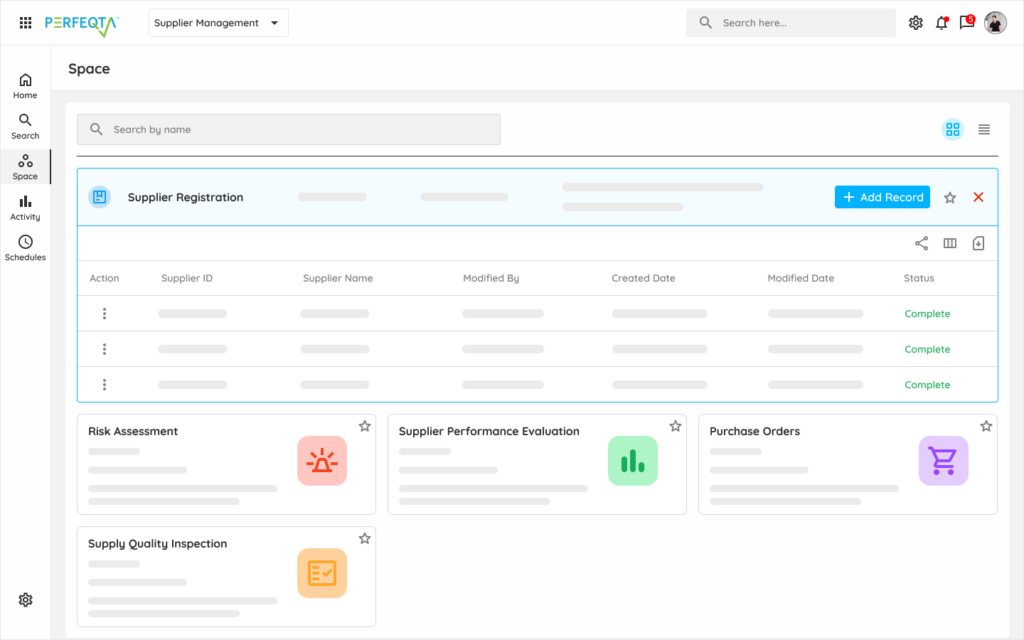
Our supplier management software simplifies supplier qualification, performance monitoring, related incidents, and audit processes.
Integrated with core quality processes such as document control, nonconformance management, and audit management, it allows you to manage all supplier records in one place.
Flexible and easy-to-use, our supplier management module enables you to streamline your supplier management processes, ensuring compliance and optimizing supplier performance.
PERFEQTA, is a risk management software that allows companies in the life science industry to consolidate their risk management files into one traceable system.
The software can link risk management documentation with products, suppliers, customers, and equipment, and provides dashboards for an accurate representation of risk across products and processes.
PERFEQTA streamlines the risk control process by creating a risk management plan and hazard analysis as part of the design control process, and enables users to stay in compliance with ISO 14971:2019 risk management procedures and forms.
The software helps develop risk management habits across the organization by creating and maintaining Risk Management SOPs that capture training records for employees.
PERFEQTA also offers additional modules for training management, CAPA management, complaint management, change management, design control, document control, equipment calibration, electronic signatures, product lifecycle management, audit management, deviation management, supplier management, form management, electronic batch records, and nonconformance management.
What our customers say about us
Experience the benefits of a comprehensive and integrated CAPA management system. Let us help you take your quality management processes to the next level.











